Largest and longest studies of any FDA-approved treatment for GA
Slows GA progression with an increasing effect over time. The greatest difference was seen during the last 6 months of the 2-year studies
SYFOVRE was studied in 2 clinical studies with over 1200 people.
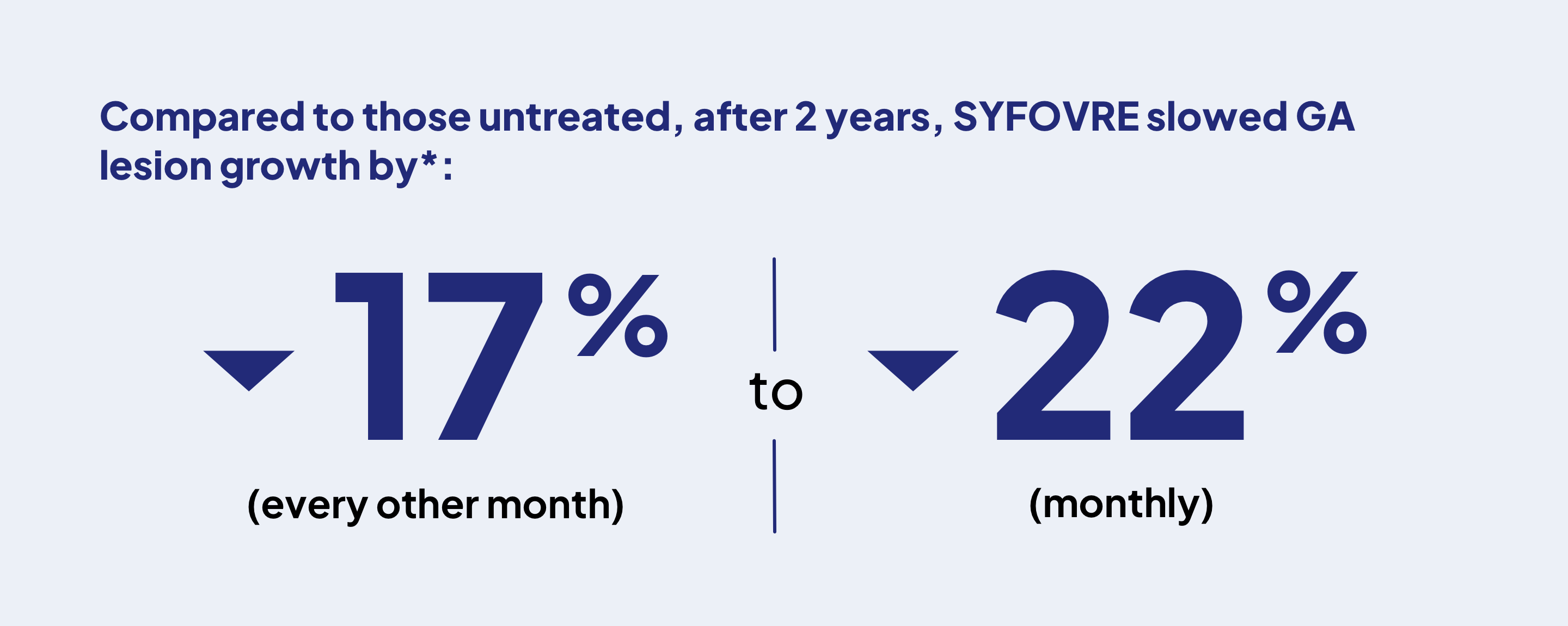
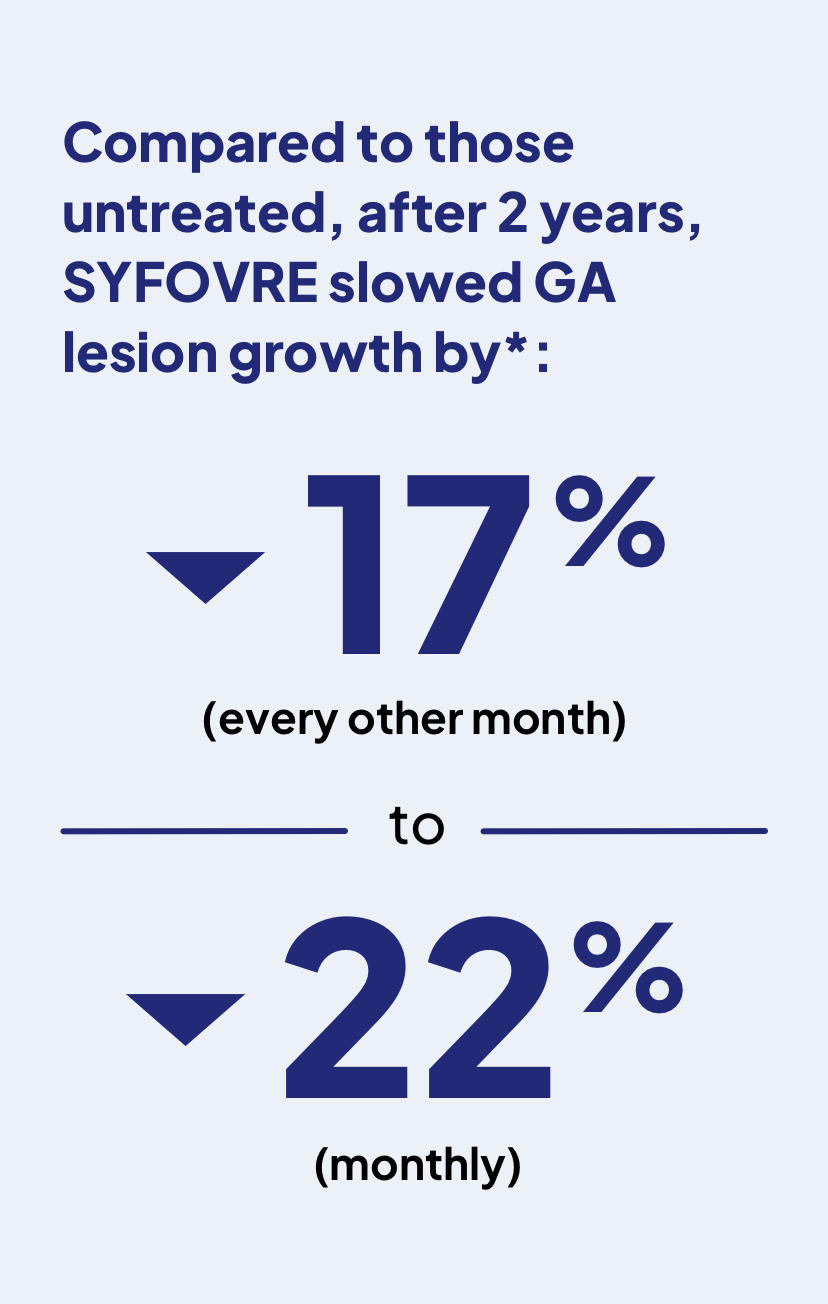
*After 2 years, SYFOVRE slowed GA progression by 18% and 22% (monthly, 403 people) or by 17% and 18% (every other month, 406 people) compared to those untreated.
SYFOVRE in a long-term study
About the long-term study
After the 2-Year studies, 508 previously treated people continued on SYFOVRE in a long-term study (monthly: 241 people; every other month: 267 people).
- The long-term study is ongoing but results from the first year are available now. Combined with the results from the 2-year studies, these show results of people who took SYFOVRE for a total of 3 years
- In the long-term study, because everyone receives SYFOVRE, lesion growth in people treated with SYFOVRE is compared to the estimated lesion growth based on the average seen in untreated people from the 2-Year studies
- These analyses for the first year of the long-term study use a projected lesion growth rate, which may not fully reflect how the condition progresses in all people with GA. Because of how the study was designed and the way the results were tested, this data should be interpreted with caution and no conclusions can be drawn
Long-term study results
Results from the first year of the long-term study
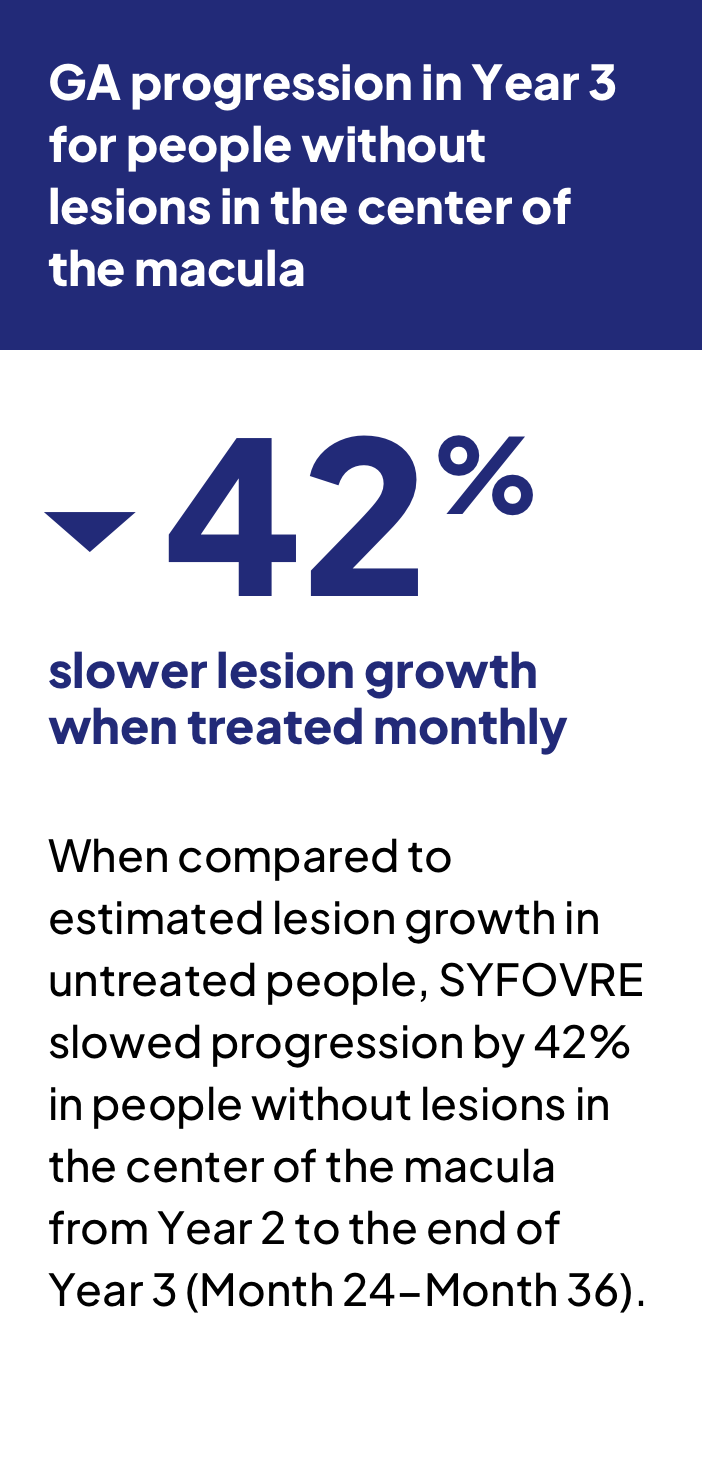
When compared to an estimated lesion growth in untreated people, SYFOVRE slowed GA progression by 25% (monthly) and 20% (every other month) from the beginning of the studies to the end of Year 3. From Year 2 to the end of Year 3 (Month 24-Month 36), SYFOVRE slowed GA progression by 35% (monthly) and 24% (every other month).
Results in people with and without lesions in the center of the macula
The clinical studies included people who had lesions in the center of their macula and those who did not. The results from these two groups were analyzed to see how SYFOVRE affected them.
Compared to estimated lesion growth in untreated people from the beginning of the studies to the end of Year 3:
- SYFOVRE slowed lesion growth by 21% (monthly) and 19% (every other month) in people whose lesions had progressed into the center of the macula at the beginning of the studies
- SYFOVRE slowed lesion growth by 32% (monthly) and 26% (every other month) in people whose lesions had not progressed into the center of the macula at the beginning of the studies








 Back to top
Back to top